Research
I am interested in the development of novel therapeutics, specifically in drug transporter mechanisms. Furthermore, I’m enticed by the unique intersection of physiology, molecular biology and pharmacology that composes pharmacokinetic research.
Previous Research Experience
In 2019, I participated in ASPIRE, a summer undergraduate research experience with The Ohio State University’s College of Medicine. The ASPIRE Medical Research Program is an initiative of the Medical Scientist Training Program (MSTP) designed to support undergraduate students interested in pursuing a professional or graduate degree in the biosciences. ASPIRE fully funds students for 10 weeks to conduct research with a principle investigator (PI).
Under the supervision of Dr. Elizabeth Kirby, I conducted over 400 hours of research investigating neural stem and progenitor cells (NSPCs). Dr. Kirby’s lab investigates how NSPCs influence the adult brain, hypothesizing that NSPCs primairly influence brain development through secreted growth factors1, such as vascular endothelial growth factor (VEGF).
During my time at the Kirby lab, I extended this research by investigating cell-specific inducible knockdown of MetRS-ANL staining in vivo. Cell-specific protein tagging would allow for identification of specific cells that secrete growth factors. Bioorthogonal tagging utilizes noncanonical amino acids (NCAAs) to tag proteins in vivo2. These noncanonical amino acids NCAAs are incorporated into protein synthesis2, which allows the tagged proteins to be visualized using microscopy or in gel florescent imaging2.
The methionine-tRNA synthetase 1 (Mars1) gene in mice encodes for the methionine-tRNA ligase3, which catalyzes the binding of methionine to the tRNA complex to produce L-methionyl-tRNAMet4. Mutating the Mars1 gene produces a mutated methionyl-tRNA synthestase (MetRS*)5 that binds to the noncanonical amino acid azidonorleucine (ANL)6, whereas an unmutated Mars1 gene produces a MetRS that cannot bind to ANL5.
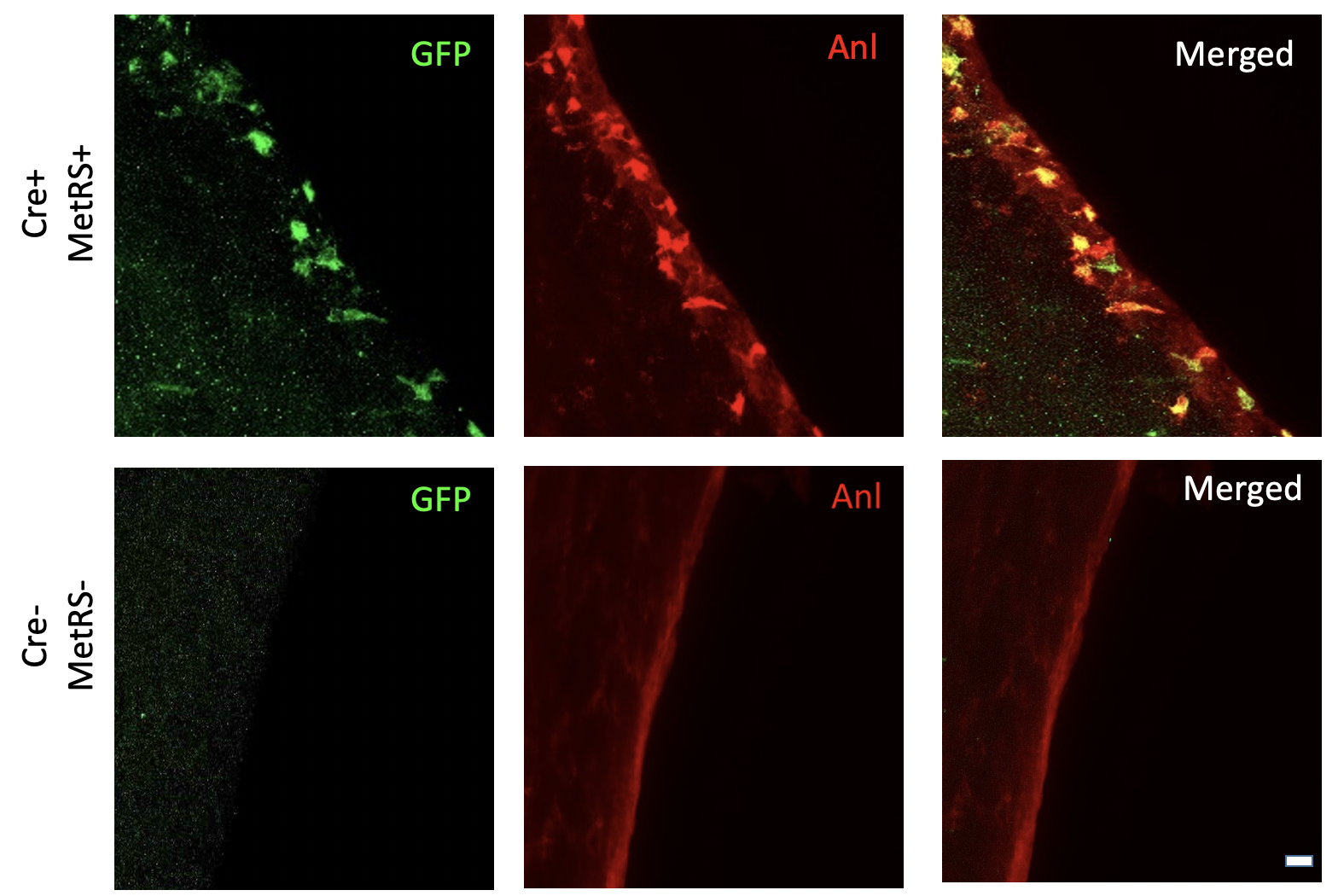
By utilizing the Nestin Cre/ERT2 MetRS Stopped Flox mouse model developed, an experiment was conducted to determine if intraperitoneal injections of noncanonical amino acids systematically enter the brain in mice. The mice were fed a low-methionine diet for 15 days. Starting on the 8th day, the mice were administered tamoxifen for 5 days. The following day, on the 9th day of the diet, IP ANL injections were administered for 6 days. On the 15th day of the experiment, the mice brains werre perfused and harvested. As depicted in Figure 1 above, ANL-tagged proteins were detected through immunofluorescence imaging GFP+ cells in the subventricular zone.
However, recombination was not present in the dentate gyrus. Subsequently, ANL-tagged proteins were detected in the subventricular zone but not the dentate gyrus by in gel fluorescence. The experiment was repeated again with the same procedure, resulting in similar results. ANL-tagged proteins were detected in the subventricular zone, but not in the dentate gyrus or cortex by in gel fluorescence.
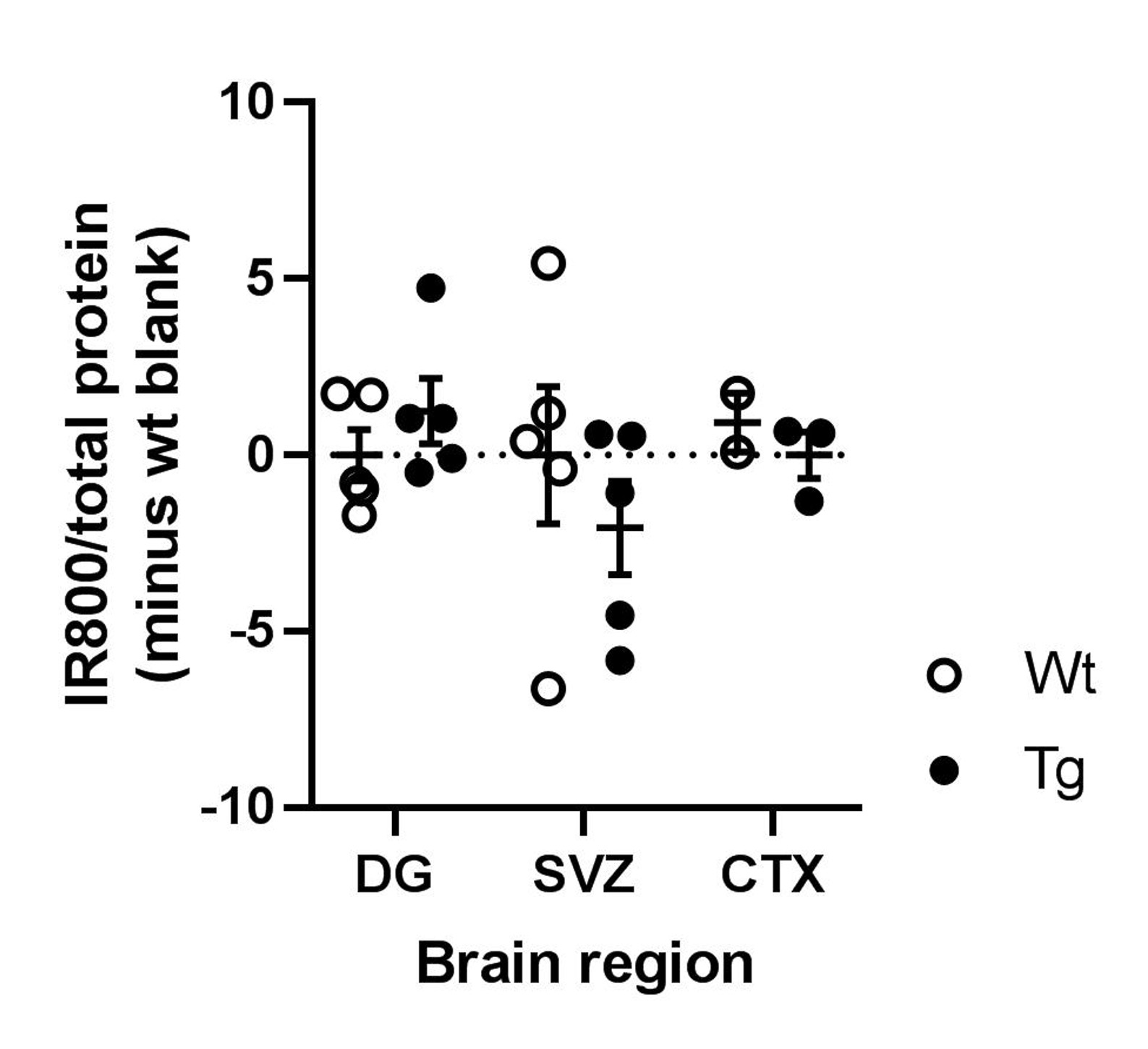
Significant differences among quanity of ANL-tagged proteins between wild-type and MetR+ positive mice were not detected. Figure 2 summarizes the protein quanities determined through in gel fluorescence. This marked the end of my research as the summer undergraduate research program concluded.
References
- Kirby, E. D., Kuwahara, A. A., Messer, R. L., & Wyss-Coray, T. (2015). Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proceedings of the National Academy of Sciences of the United States of America, 112(13), 4128–4133. https://doi.org/10.1073/pnas.1422448112
Wier, G. M., McGreevy, E. M., Brown, M. J., & Boyle, J. P. (2015). New method for the orthogonal labeling and purification of Toxoplasma gondii proteins while inside the host cell. mBio, 6(2), e01628. https://doi.org/10.1128/mBio.01628-14
- Ibba, M., & Soll, D. (2000). Aminoacyl-tRNA synthesis. Annual review of biochemistry, 69, 617–650. https://doi.org/10.1146/annurev.biochem.69.1.617
- Lee, C. P., Dyson, M. R., Mandal, N., Varshney, U., Bahramian, B., & RajBhandary, U. L. (1992). Striking effects of coupling mutations in the acceptor stem on recognition of tRNAs by Escherichia coli Met-tRNA synthetase and Met-tRNA transformylase. Proceedings of the National Academy of Sciences of the United States of America, 89(19), 9262–9266. https://doi.org/10.1073/pnas.89.19.9262
- Alvarez-Castelao, B., Schanzenbächer, C. T., Hanus, C., Glock, C., Tom Dieck, S., Dörrbaum, A. R., Bartnik, I., Nassim-Assir, B., Ciirdaeva, E., Mueller, A., Dieterich, D. C., Tirrell, D. A., Langer, J. D., & Schuman, E. M. (2017). Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nature biotechnology, 35(12), 1196–1201. https://doi.org/10.1038/nbt.4016
- Tanrikulu, I. C., Schmitt, E., Mechulam, Y., Goddard, W. A., 3rd, & Tirrell, D. A. (2009). Discovery of Escherichia coli methionyl-tRNA synthetase mutants for efficient labeling of proteins with azidonorleucine in vivo. Proceedings of the National Academy of Sciences of the United States of America, 106(36), 15285–15290. https://doi.org/10.1073/pnas.0905735106
